The Trusted Standard in Research, Ethics, Compliance, and Safety Training
The Collaborative Institutional Training Initiative (CITI Program) is dedicated to serving the training needs of colleges and universities, healthcare institutions, technology and research organizations, and governmental agencies, as they foster integrity and professional advancement of their learners.
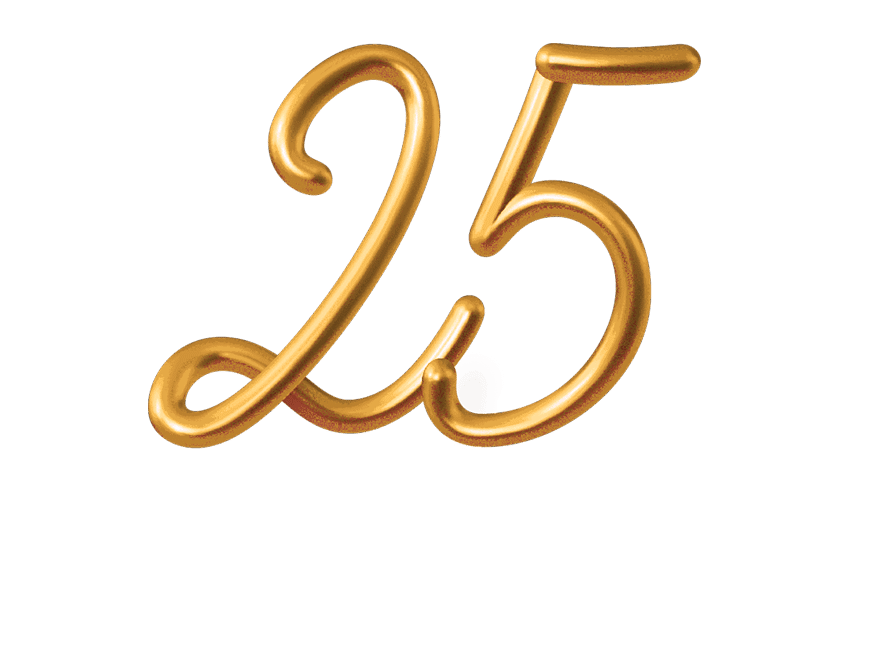



Explore Our Featured Courses
Introduces a systematic method for analyzing safety statistics

An introductory video-based series to the DOE’s SBIR-STTR funding program and a discussion of key elements of the proposal elements.

This course explores explores technological advances and eConsults.

Introduces you to key information about, and tools to use, the 3Rs of animal research.

These courses explore key topics related to research security and international engagement.

Provides learners with best practices for creating and maintaining study documents.

Covers how to identify red flags in your survey data, strategies to develop surveys to minimize fraud, and best practices for data management.

Understand CUI and the obligations it creates for universities and researchers.

Explores considerations for a human research protection program (HRPP) emergency preparedness plan.

Learn how to review nonexempt human subjects research protocols for regulatory compliance with the Common Rule.

Provides learners with key information on the management of controlled substances in clinical research settings.

This role-based course provides the practical know-how to monitor clinical research sites effectively.

Provides an introduction to the EU AI Act, including risk-based approaches, compliance obligations, enforcement, interplays with other European digital policy, and perspectives

An introduction for students to the meaning of academic integrity and plagiarism.

Explores common motives behind fraudulent survey research participation, how to address and mitigate the impact of identified fraudulent data, and how to minimize opportunities for malicious intent.

Explores strategies for remaining diligent about securing online survey research.

Examines ethical and policy issues about xenotransplantation.

Explores the challenges and opportunities emerging technologies present to homeland security, covering topics such as cyber threats, ransomware, drone misuse, IoT vulnerabilities, AI threats, disinformation, and deepfakes.

This webinar reflects on the creation of a culture of compliance through the lens of how one university created an enterprise-wide compliance role.

Discusses what events must reported, to whom, and according to what timeline.

Learn about the FCPA and its impact to healthcare operations.

This webinar offers practical solutions for optimizing corporate board roles to foster a culture of ethics and compliance.

A role-based course that provides practical know-how to effectively lead or participate on data monitoring committees.

Overview of the European Union’s (EU) General Data Protection Regulation (GDPR).

Discusses the four pillars of a culture of care, the impact of compassion fatigue, and individual and institutional strategies to address compassion fatigue.

The webinar explores work-related musculoskeletal disorders among surgical teams.

An introduction to risk management and crisis response within study abroad programs.

An in-depth review of advanced topics important to research administrators.

Stress at research institutions is common, explore ways to navigate and mitigate the effects of stress in these settings.

This course focuses on promoting the success of international students on university campuses by offering strategies for academic support, cultural integration, and compliance with U.S. federal policies.

N2 is a not-for-profit alliance of Canadian research networks and organizations working to enhance national clinical research capability and capacity.

BIC Study Foundation is a resource for those who want to take CITI Program courses in Korean. They are a certified training provider by the Korean FDA/MAFRA for HRPP and ACU Programs.

A complete research compliance suite that supports IRB, IACUC Conflict of interest, Bio Safety Export Control, and more.

HRP Consulting provides customized services for your research program, including temporary staffing, IRB/IACUC assistance, accreditation support, program evaluations, training/education and more.

CTrials helps grow sponsored research programs by taking on the many administrative challenges inherent in managing clinical trials.

Informed Consent Builder is a cloud-based platform that streamlines the process of managing and generating informed consent forms.

Protocol Builder is an online protocol writing and collaboration platform that also speed up your pre-review turnaround times.

Join Over 2,500 Subscribing Organizations
Highlighted below are just a few select subscribers & collaborators.










Meet A Few Of Our Expert Authors and Presenters
Our courses are built by over 350 highly qualified experts and rigorously peer reviewed to incorporate various perspectives and ensure accuracy, completeness, and overall quality.

Adam Roth, MA
CTI Clinical Trial & ConsultingAdam Roth has 25+ years experience in clinical research. He has held several roles, including clinical research coordinator and manager at a research site, project Manager at a CRO, operations director at a central IRB, and is currently Vice President of Research Site Services at a phase 1-4 multi-specialty clinical research site.

Erica Heath, CIP, MBA
RetiredIn 1984, after 14 years at the UCSF CHR, I opened an independent IRB named, Independent Review Consulting (IRC). In 2010, IRC merged with ERC to create a new independent IRB named Ethical and Independent Review Services or E&I Review. I retired in 2016 and speak happily with IRB friends.

John Baumann, PhD
Indiana UniversityJohn R. Baumann, PhD was the Associate Vice President for Research Compliance, Indiana University. He is a site visitor – and former member of Council of Accreditation – for AAHRPP and chairs the IRBs of NDRI-USA and AAFP. He also provides consulting services on research compliance and responsible conduct of research.

Michael Petillo, MS
CES Partnership, LLCMichael (he/they) has worked in non-profit program management, mixed methods evaluation, organizational capacity building, grant writing, and international community development. His/their work has included Native Nation Building research, HIV/AIDS service and prevention, DEIAJ in health equity-focused research and higher education settings, LGBTQ+ and gender-based violence advocacy, and creative writing.
In Our Learners' Words

Maria E.
Evidence Based Practice Nursing Resident, Diabetes Nurse Specialist
Great information on the importance of protections in research with human subjects. I liked the audiovisual option.

Myrene M.
Associate Faculty
The course materials are brief and clear. Such brevity and clarity are perfect for those who are busy with other duties at work and provide for a more friendly and less overwhelming task. I love the quizzes because I can learn from my mistakes. After submitting the quiz, the correct and wrong answers are explained. This reinforces learning.

Tonya W.
Middle School Related Arts Teacher
I liked the video case studies the best. They made the provided information more personable and helped course sections make more sense.

Alyssa I.
PhD Student
The variability in content (lectures, videos, case studies) helped me understand the material better.

Courses Approved by Top Continuing Education Accreditors
CITI Program courses are approved for CME credits through the Albert Einstein Montefiore Continuing Professional Development Center (CPDC). Albert Einstein College of Medicine-Montefiore Medical Center (Einstein) is accredited by the Joint Accreditation for Interprofessional Continuing Education to provide continuing education activities for healthcare professionals. Einstein is accredited to offer continuing education credit for the following professions: medicine, nursing, psychology, pharmacy, dentistry, optometry, social work, nutritional science, and athletic training.
CITI Program is also accredited by the International Accreditors for Continuing Education and Training (IACET) and offers IACET CEUs for its learning events that comply with the ANSI/IACET Continuing Education and Training Standard. IACET is recognized internationally as a standard development organization and accrediting body that promotes quality of continuing education and training.
Recent News & Articles

Additional TelehealthVillage Courses for Diabetes and Other Specialties
These courses are designed to empower healthcare professionals with the skills to deliver innovative, patient-centered care through the power of virtual technology.
Read the article
CITI Program Funding Finds – April 16, 2025
“CITI Program Funding Finds” is our monthly blog series which highlights opportunities relevant to research grants, funding, programs open to new applicants, and more. Follow...
Read the article
Free Live Webinar – ARRIVE Guidelines for Reporting Animal Research
This webinar addresses the critical issue of reproducibility in preclinical research.
Read the article